Without exaggerating the importance of humidity problems in our temperate climate, we have understood the inconvenience of air which is too humid or too dry inside rooms.
For reasons of airing and ventilation, interior humidity is influenced by humidity levels outside. Before looking at the treatment of interior humidity, we are going to deal with that outside.
Let us recall that (E-book Temperature, expansion in HVAC ”):
- A body in liquid or gas state is composed of the same molecules (H2O for water and for vapour). There is simply more molecule agitation in the gas state compared to the liquid state.
- In a given state, solid, liquid or gas, the molecules are more agitated when the temperature is high.
- Rain is composed of water drops that are sufficiently heavy to fall towards the ground.
- Fog or clouds are composed of miniscule water drops (and not vapour) suspended in the air.
- Vapour is invisible.

Question
What do we see in the kitchen above the boiling sauce-pan ?
Water vapor or water in suspension ?
Water vapor is invisible; the “cloud” we see above the sauce-pan is composed of minuscule water droplets!
In fact, if we look close to the surface of the water we see nothing, since this is where the vapor is. But, coming rapidly into contact with the colder air in the kitchen, the molecules of vapor “settle down” and group together on condensing.
Clouds are formed exactly in the same way. We don’t see the “tonnes of vapor” rising above lakes, rivers and the sea, but at altitude this vapor cools and condenses, leading to the formation of clouds.Look carefully at the top of large boiler chimneys. You can see that the most of the “smoke” is not formed at the chimney outlet. This area seems to be relatively free because the escaping vapour has not yet condensed.

Let us suppose that the air is hot and humid. It contains many invisible vapour molecules, which are very agitated because of the “high” temperature. If we cool this air the molecule agitation will decrease and certain of them will transform into liquid state.
They will then group together to form fog or rain.
We can therefore understand that hot air can contain many more agitated vapour molecules than cold air in which the same “calmer” molecules can easily transform into a liquid state.
We can now understand why in temperate climates it rains more in winter than in summer, and that fog forms in the night when it is cold.
Morning mists will often disappear towards midday when the sun has heated the air sufficiently for the water molecules to recover the agitation necessary to revert to gas state.
In conclusion:
- Hot air can contain a lot of vapour
- Cold air cannot contain a lot of vapour
Consequently:
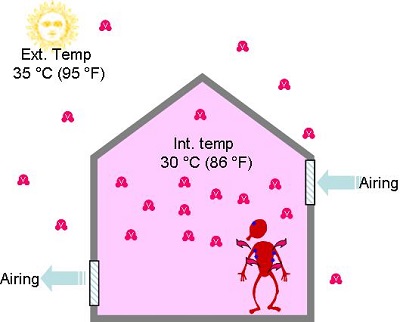
- In winter when we introduce outside air to air buildings, it contains very little vapour and will feel dry to the occupants
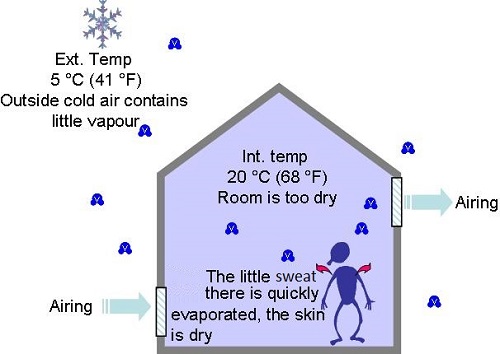
- In summer, when we introduce outside air to air buildings, it contains sometimes lots of vapour and will feel humid to the occupants.