Energy allows movement.
The
International unit for energy is the joule
which
corresponds to the raising of a weight of 1 Newton (a weight ≈ 100
grammes) by a height of 1 [m].
Energy,
“displacement of a weight (a force) by a height”, is a physical
value composed of length, mass and time.
The
Joule is the name given to [kg.m2/s2]
All
energy can be converted into heat.
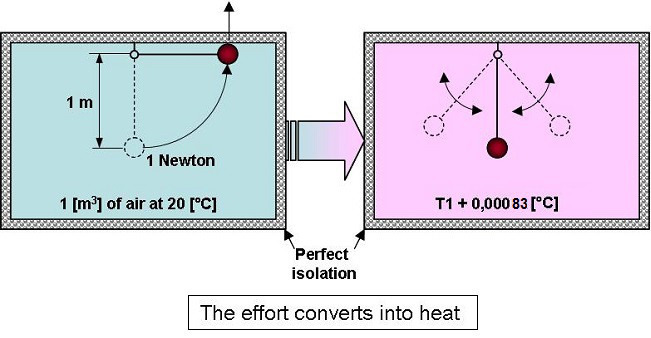
If an object whose weight is 1
newton, placed within a perfectly isolated volume of 1 [m3]
of air
at
20 [°C] (68°F), is gently raised by 1 [m], then dropped, the
temperature will rise by 0.00083 [°C]… (0.00083 [K]).
In fact,
when everything comes back to rest, the air will have received 1
joule of energy which will be converted into heat.
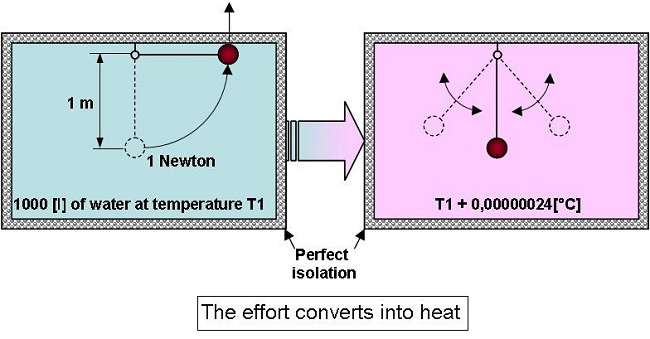
If
an object whose weight is 1 newton, placed within a perfectly
isolated volume of 1 [m3]
of water
at 20 [°C] (68°F), is gently raised by 1[m], and then dropped, the
temperature will rise by 0.000,000,24 [°C]… (0.000,000,24 [K]).
In
fact when everything comes back to rest the water will have received
1 joule of energy which will be converted into heat.
The joule can thus be used to quantify quantities of heat, but is a unit which is extremely small for our branch of activity. We will therefore tend to use:
- The kilojoule: [kJ] = 1,000 [J] (0.95 Btu)
- The megajoule: [MJ] = 1,000,000 [J] (948 Btu)
The symbol for heat is the letter: Q
