Heat is a form of energy exchanged between two bodies of different temperatures.
This heat exchange occurs in the form of microscopic mechanical energy, corresponding to the degree of agitation of the molecules. When a body receives heat, its molecular agitation tends to intensify, usually resulting in an increase in temperature.
However, an introduction of heat can also result in a change of state: if we heat an ice-cube, it gradually melts while remaining at 0 [°C] (32°F).
Heat
spreads spontaneously from the
hotter body to the cooler body,
thus raising the temperature of the cooler body and lowering its
own.
Heat will only transfer in reverse (i.e. from a cooler body
to a hotter one) if additional energy is supplied to achieve what is
an unnatural transfer.

If the hot body is in contact with the cold one, the heat is transferred from one to another through conduction.
The surface molecules of the hot body will lose part of their agitation to the benefit of the surface molecules on the cold body.
An unbalanced agitation will result on the surfaces of both the hot and cold bodies, which will be interchanged between the two bodies until they have reached the same average temperature.
If
the bodies which are in contact are fluids, the “heated”
molecules will generally rise because of their expansion, and the
“cooled” molecules will descend.
This type of heat conduction
is called convection.
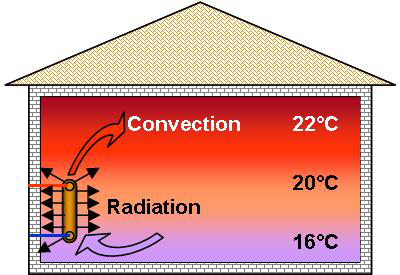
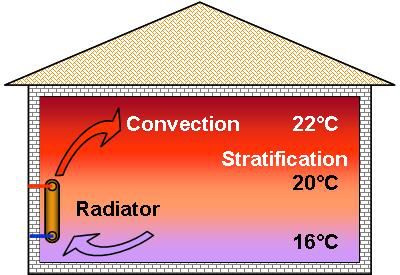
Hence,
above a radiator, we feel the heated air rise through convection. In
the heated room, the air will always be warmer nearer the ceiling
than at floor level. This phenomenon is called stratification.
If
the hot body is not in contact with the cold body the
transfer of heat is carried out by radiation.
It
is by this form of transfer that the sun heats the earth. Energy is
transferred in the form of elementary mass-less particles called
photons. When these photons reach the surface of a cold body, they
agitate the molecules.
Once there is a temperature difference
between 2 bodies which are not in contact, the exchange is by
radiation.
The radiator thereby transfers its heat to the air through convection and radiation to all the cooler surrounding surfaces. The proportion between these 2 forms of exchange depends on the type of radiator.